Over 25 years of industry-wide cell therapy research has resulted in once-inconceivable treatment options for patients with serious blood cancers who previously had few choices. Ten years after the first generation of autologous CAR T cell therapies, the fruits of that research are being seen, as patient stories renew hope.
Today's cell therapy insights shaping tomorrow
While cancer remains one of the greatest global health challenges, there have been unprecedented breakthroughs and innovations in cancer research in recent years, and more people are living longer with cancer than ever before.
This year, Bristol Myers Squibb leaders have met with peers like Carl June, a renowned pioneer in cell therapy, to discuss the latest in the field.
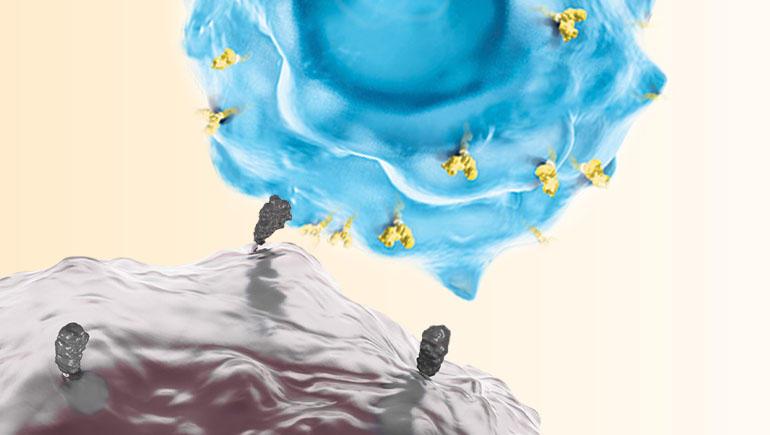
One innovation changing the way certain cancers can be treated and offering hope to patients and physicians around the world is cell therapy, a type of cancer immunotherapy where a patient’s own T cells are genetically engineered to recognize and bind to proteins found on the surface of certain cancer cells. Binding then leads to activation and expansion of the CAR T cells, resulting in the killing of cancer cells.
Read on to discover some key insights that help Bristol Myers Squibb press forward in this increasingly important field.
Subscribe to Our stories alerts
Beyond just relevant information about Bristol Myers Squibb's therapeutic areas and innovation, Our stories offer a window into the work our employees do every day for patients.
However, the industry has only scratched the surface of cell therapy’s potential impact for patients with cancer. Globally, cancer ranks as a leading cause of death and an important barrier to increasing life expectancy — in 2020, there were an estimated 19.3 million new cases of cancer. The challenge of filling this unmet need is motivating and a reminder of what there still is to accomplish.
One thing is clear: riding this wave of excitement in the field will help unlock its potential for more patients.
As in the early days of any scientific field, scientists and other stakeholders in cell therapy still face an uphill climb. Cell therapy is a personalized medicine, and the process to get each treatment to patients is complex and intricate. Scaling operations remains a galvanizing challenge and a major focus of players across the industry.
Next-generation cell therapies, including allogeneic, or “off-the-shelf” approaches where CAR T cells are derived from healthy donors or induced pluripotent stem cells (iPSCs), have the potential to mitigate operational challenges by removing the initial step in which a patient’s cells are harvested for CAR T manufacturing. But unknowns remain — the science is so new that we are only starting to gather data on the durability of this approach, which will make it a key priority of research moving forward.
Translating cell therapy science to treat solid tumors has also presented challenges, and researchers are actively exploring new approaches and targets that will extend the promise of cell therapy beyond blood cancers. There is potential for a long-term sustainable therapy in these diseases with the use of an auto-antigen as the external chimeric binder on the CAR T cell.
Bristol Myers Squibb is working to see around the bend and collaborate with industry peers and stakeholders to anticipate and tackle these hurdles.
Development programs at Bristol Myers Squibb are centered around patient needs, but cell therapy requires additional consideration, as the medicine itself originates from the person being treated. Patients are at the center of every decision the company makes, from the design of clinical trials to how patients receive treatments in the real world.
Certain strategies can help make the cell therapy treatment experience easier for patients. Depending on a person’s health, some cell therapies can be administered in the outpatient setting, allowing the individual to recover at home while being closely monitored by his or her care team post-infusion. In addition, technology such as the TempTraq® wearable temperature-monitoring device offered to Bristol Myers Squibb CAR T patients can assist patients and caregivers with temperature monitoring outside the hospital setting during the post-infusion monitoring period.
While the industry looks ahead to next-generation cell therapies, it's imperative to continue working to optimize the current therapies available to patients. Beyond clinical trials, Bristol Myers Squibb development teams work closely with manufacturing teams to use technology and automation to continually improve the process, assessing which new approaches can be translated from the lab to the manufacturing floor.
It is equally important that this new modality is brought to patient populations reflective of the real-world need. As the company scales operations, in parallel Bristol Myers Squibb is working to make cell therapies available to underrepresented patient groups, and that starts with clinical trials. Development teams within the company are working to address trial diversity, which is critical for equitable innovation.
What these insights mean to Bristol Myers Squibb
Transforming patients’ lives through science remains the company’s focus. Playing a leadership role in the ongoing advancement of this field is one of the ways the company strives to help those living with cancer. The best is yet to come in cell therapy and Bristol Myers Squibb is committed to continue to learn, grow and invest in its progress.
| To learn more about cell therapy, please visit our Cell Therapy Resources page. |
About Bristol Myers Squibb
Bristol Myers Squibb is a global biopharmaceutical company whose mission is to discover, develop and deliver innovative medicines that help patients prevail over serious diseases. As global citizens, we work sustainably and responsibly to create a positive impact in the communities where we live and work.