Exploring multiple paths to advance BCMA-targeted therapies
More than 275,000 new cases of multiple myeloma are expected to occur globally by the year 2040. Multiple myeloma is a blood cancer characterized by the proliferation of malignant plasma cells that typically originate in the bone marrow. B-cell maturation antigen (BCMA) is an important cell surface antigen found in nearly all cases of multiple myeloma, but with limited expression on normal tissues other than plasma cells. This makes it a key target for multiple myeloma therapeutics.
For over two decades, Bristol Myers Squibb has been working to advance the treatment of multiple myeloma by investigating the molecular origins of the disease and to develop therapies across a range of modalities which take advantage of those targets. Multiple myeloma, however, remains an incurable disease. Patients endure cycles of relapse and remission, receiving treatments that offer the hope of better outcomes but still can fail them. All patients deserve a tailored treatment approach to achieve the best possible outcomes, so it is critical that novel therapies across multiple and varying modalities are continuously brought forth to provide new options for patients.
Bristol Myers Squibb is developing therapies targeting BCMA by leveraging a number of modalities including cell therapies, antibody-drug conjugates and immune cell engagers. The company’s BCMA portfolio is broad and teams are evaluating different approaches by investigating multiple BCMA-targeted therapies.
Targeting BCMA using various advanced modalities allows the investigation of novel combinations with the hope that ultimately, cancer treatments can reflect what is best suited for each individual patient. This forward progress is informed by industry-leading data sets and application of translational learnings across programs for optimized patient selection, process improvements and pipeline acceleration.
Research approaches include:
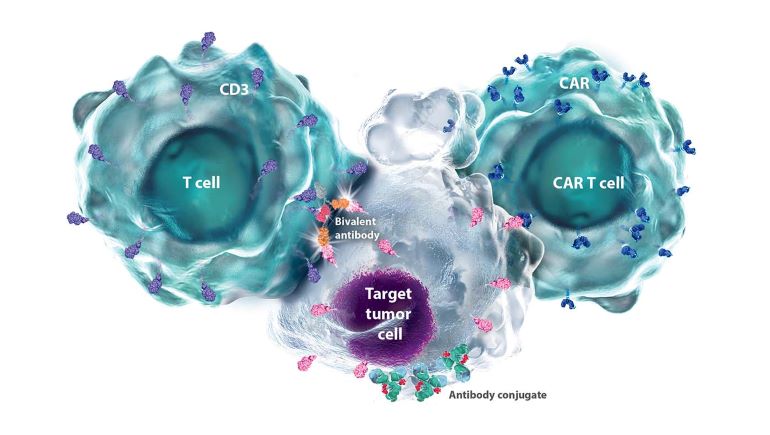
- Chimeric antigen receptor (CAR) T cell therapies: CAR T cell therapy is a personalized approach to treating certain blood cancers. The therapy is made from a patient’s own T cells, reprogramming them to recognize and bind to proteins (tumor-associated antigens, TAAs) found on the surface of certain cells, including cancerous cells. Bristol Myers Squibb is advancing multiple CAR T cell therapies targeting BCMA with the aim of making autologous CAR T cell therapies more effective, safe and accessible while improving upon efficiency and scalability.
- Immune cell engagers: These engineered multi-specific biologic molecules have one side that binds to one or more TAAs and another side that binds to receptors on the surface of an immune cell (e.g., T cells or NK cells). This binding redirects the patient’s immune cells toward cancer cells with the goal of triggering cancer cell death. Immune cell engagers represent an “off-the-shelf” treatment option that leverages the patient’s own endogenous immune cells without the complexity of ex vivo cell engineering. Bristol Myers Squibb is advancing multiple immune cell engagers directed at BCMA.
Additionally, BMS is studying antibody-drug conjugates (ADCs) in early stages, which operate by tethering a small molecule to a monoclonal antibody, and are engineered to deliver cytotoxic payloads to targeted locations using antibodies as tumor-homing mechanisms resulting in cancer cell death. BMS researchers are targeting the cell surface antigen BCMA to direct ADCs to kill cancer cells.
Bristol Myers Squibb continues to advance research to unlock the full potential of BCMA-targeted therapies across approaches while also advancing therapies using other novel targets, such as G-protein coupled receptor class C group 5 member D (GPRC5D). GPRC5D is expressed on most multiple myeloma cells independently of BCMA and can be used as a second target in multiple myeloma in patients who may not respond to or progress following receipt of BCMA-directed therapies.
Beyond targeting BCMA, Bristol Myers Squibb pioneered the development of novel CELMoDTM agents for the treatment of multiple myeloma. CELMoD agents promote protein degradation to remove disease-causing proteins from cells. Specifically, these molecules act as “molecular glue” by altering the protein-binding properties of cereblon (an E3 subunit and an important component of the protein degradation cellular machinery) to promote interaction with and degradation of disease-causing proteins.
Research at Bristol Myers Squibb is propelled by a rich history of developing life-changing medicines and an unwavering commitment to follow the science and push forward when it matters most. Bristol Myers Squibb is equipped to innovate, now and in the future, to continue to bring forth new treatment options backed by decades of unparalleled experience.
Subscribe to Our stories alerts
Beyond just relevant information about Bristol Myers Squibb's therapeutic areas and innovation, Our stories offer a window into the work our employees do every day for patients.
About Bristol Myers Squibb
Bristol Myers Squibb is a global biopharmaceutical company whose mission is to discover, develop and deliver innovative medicines that help patients prevail over serious diseases. As global citizens, we work sustainably and responsibly to create a positive impact in the communities where we live and work.